Primary pollutants and secondary pollutants can be more dangerous. The first are those that are emitted directly from a source, which can be natural (volcanic eruptions or fires, for example) or of anthropogenic origin (carbon monoxide from vehicles).
Secondary pollutants, on the other hand, are not emitted directly. Its origin lies in the interactions between the primary emissions in the atmosphere. One of the most known secondary pollutants is tropospheric ozone, whose formation and effect will be explained in the following section.
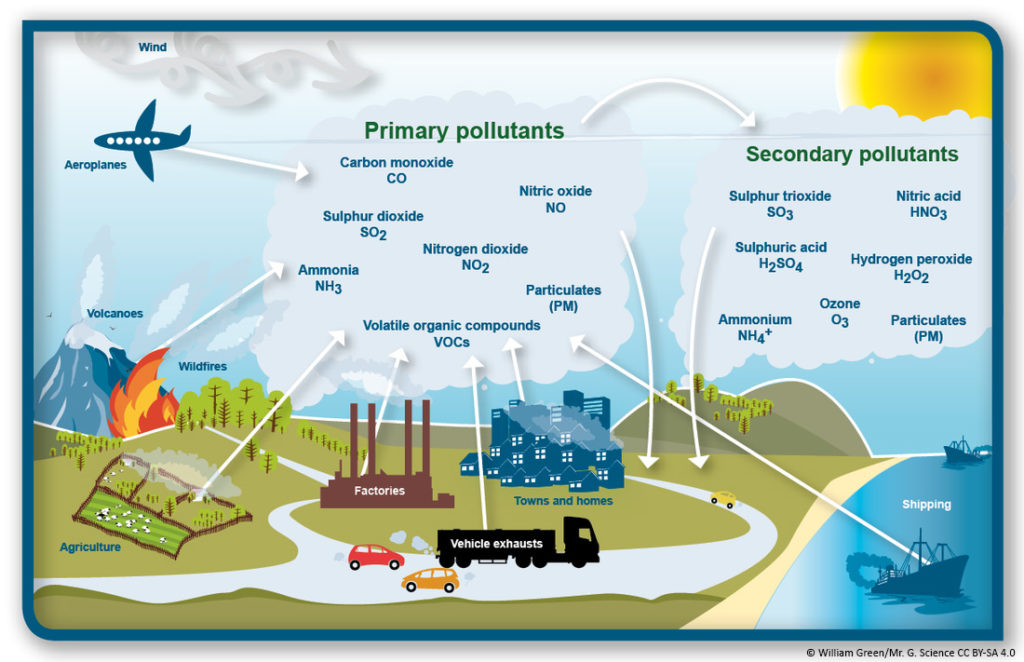
What are the most dangerous primary and secondary pollutants?
Understanding the details about how pollutants are produced, how they are transformed once they are emitted and the danger they can represent, it is important for designing minimization-oriented measures.
Primary pollutants, the beginning of the chain
The main primary pollutants and their effects are:
- Carbon monoxide (CO), the result of the incomplete combustion of organic matter, which is why one of the main sources of emission is the associated traffic and the burning of fossil fuels. It is a flammable gas that is toxic to people even in small concentrations. It is a precursor of CO2 and ozone.
- Sulfur dioxide (SO2), which reaches the atmosphere mainly as a consequence of human activities such as the burning of coal or oil. Natural sources such as volcanoes also contribute a remarkable percentage. Its main danger is its subsequent transformation into sulfuric acid (H2SO4), which causes acid rain.
- Nitrogen oxides (NOx), a name that includes nitric oxide (NO) and nitrogen dioxide (NO2). Its main source is motorized vehicles, although fires and volcanoes also emit nitrogen compounds into the atmosphere. It is one of the main causes of smog, also giving rise to acid rain when it is transformed into nitric acid.
- Ammonia (NH3), a flammable, toxic and burnt gas that has an important emission focus in agricultural activity as a result of the use of fertilizers. It is also, as can be seen on the website of the European Environment Agency, the only pollutant whose generation remains stable.
- Particles in suspension (PM), constituted by dust, pollen, ashes, metallic particles, etc. Their dangerousness depends on their size, since the smaller particles can get to be absorbed by the blood. They can be, therefore, the vehicle of entry into the human body of numerous harmful substances.
- Volatile organic compounds (VOCs), formed by hydrocarbons in a gaseous state at room temperature. They are toxic substances that give rise to photochemical oxidants such as ozone.
- Heavy metals, not included in the previous image, but which represent a high danger due to their cumulative power and their absence of degradation in nature, such as lead (Pb) and mercury (Hg). They originate mainly in combustion facilities, cement or glass production or waste incineration facilities.
Secondary pollutants, when the atmosphere becomes a laboratory
As discussed in the introduction, the secondary pollutants result from the interaction of the primary pollutants once emitted into the atmosphere. Among the best-known substances, ozone and the compounds that give rise to acid rain, which will be the focus of this section.
Tropospheric ozone or “bad ozone” is formed by the interaction of various precursors (volatile organic compounds, CO, NOx, etc.) in the presence of sunlight. Unlike stratospheric ozone, which protects the planet from the ultraviolet radiation of the sun, this ozone is dangerous for human health because in high concentrations can cause respiratory problems or eye irritation. It also has a detrimental effect on the environment, damaging crops and plants, as it slows the process of photosynthesis by reducing the absorption of CO2 by the plant.
It is the main compound of smog, a type of photochemical fog in which NOx, hydrogen peroxide, particles of nitric and sulfuric acid, etc. are also found, and which is responsible for the “pollution beret” that many cities and towns show.
The process of acid contamination
Acid contamination occurs when the soil and water undergo an acidification process, that is, when the pH is below 7 (the optimum pH for most plants, for example, oscillates between 5.5 and 7.0). However, it is not an exclusive process of the natural environment, since in urbanized areas it contributes to the so-called “stone disease”, which manifests itself in the superficial erosion of buildings. This alteration is the result of the deposition in the form of acids of SOx and NOx.
Although it is a problem that has seen a visible improvement in several areas of the planet, according to a 2018 article in the New York Times, the adoption of corrective measures and their effectiveness has not developed equally. Countries such as India and monuments of world heritage such as the Taj Mahal are a faithful example of the damage caused by these compounds.










